MyoPacer
To accommodate the needs of the myocyte research community, IonOptix developed an easy-to-use stimulator, specifically designed for pacing cardiac myocytes within our Calcium and Contractility Systems. A digital stimulator display allows users to set voltage, pulse duration, and frequency accurately and reproducibly. Output voltage, frequency, and duration can all be easily adjusted from the front panel and can be pre-programmed or changed on the fly. In addition to user-defined frequencies the MyoPacer can also be triggered using an external pulse generator. The MyoPacer’s ability to emit bipolar stimuli or to alternate stimulus polarity greatly reduces problems with electrolysis at the electrodes.

MyoPacer EP
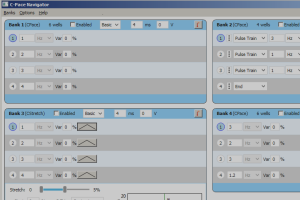
The MyoPacer EP adds cardiac electrophysiology functionality. It was created to give researchers the capability of delivering pre-designed stimulation sequences for obtaining restitution curves and doing arrhythmia and defibrillation studies. It is based on the original MyoPacer and retains all of the original functionality, but adds the ability to generate offbeats, delays, and multiple frequency protocols. The MyoPacer EP is designed around the strategy of creating multi-phase protocols.
Each protocol can have up to 5 phases. A phase can be defined either as a pulse train or a delay. If it is defined as a pulse train, a period or frequency must be selected.
Phases end either after a programmed time / no. of pulses, or when a manual or external TTL trigger is received. The end of a phase will immediately initiate the next phase or, if on the last phase, return to the first phase. As traditional electrophysiological researchers have used the terminology S1, S2… to describe the various phases, the MyoPacer EP will automatically display the appropriate label when looking at a phase.
Resources
- IonOptix Video Tutorials: Murine Cardiomyocyte Isolation
- IonOptix Video Tutorials: IonWizard Experiment Design
- IonOptix Video Tutorials: Calcium & Contractility System Acquisition Quick Start
- IonOptix Video Tutorials: CytoSolver Batch Analysis
- IonOptix Video Tutorials: Analyzing Unmarked Events
- IonOptix Video Tutorials: IonWizard Data Analysis
- Hypertrophic cardiomyopathy in high-fat diet-induced obesity: role of suppression of forkhead transcription factor and atrophy gene transcription.
- Testing MyoPacer Outputs
- MyoPacer Manual
- MyoPacer EP Manual
- Techniques and Best Practices for Cardiomyocyte Isolation
- Mechano-regulated tenascin-C orchestrates muscle repair.
Note: This product is intended for research purposes only. It is not certified for clinical applications (including diagnostic purposes). Use of this product in uncertified applications is in violation of FDA regulations.