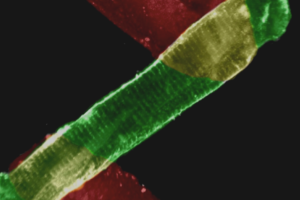
NOTE: In the ductus arteriosus of fetal mice: (this is one of the first papers to ever
use pressurized myography to study the mouse ductus, and is particularly
cool for studying premature fetal vessels).
Although prostaglandin E2 (PGE2) vasodilates the ductus arteriosus, tocolysis with cyclooxygenase (COX) inhibitors delays postnatal ductus arteriosus closure. We used fetal mice and sheep to determine whether PGE2 has a role in the development of ductus contractility that is distinct from its function as a vasodilator. Prolonged exposure of fetal ductus to PGE2 in vitro increased the expression of CaL- and K+-channel genes (CaLalpha1c, CaLbeta2, Kir6.1, and Kv1.5, which regulate oxygen-induced constriction) without affecting the genes that regulate Rho-kinase-mediated calcium sensitization. Conversely, chronic exposure to COX inhibitors in utero decreased expression of CaL- and K+-channel genes, without affecting Rho-kinase-associated genes. Chronic COX inhibition in utero decreased the ductus’ in vitro contractile response to stimuli that use CaL- and K+-channels (like O2 and K+), whereas the response to stimuli that act through Rho-kinase-mediated pathways (like U46619) was not significantly affected. Phosphodiesterase expression, which decreases the ductus’ sensitivity to cAMP- or cGMP-dependent vasodilators, was increased by PGE2 exposure and decreased by COX inhibition, respectively. These studies identify potential downstream effectors of a PGE2-mediated, developmental program, regulating oxygen-induced ductus closure. Alterations in these effectors may explain the increased risk of patent ductus arteriosus (PDA) after in utero COX inhibition.
Chronic inutero cyclooxygenase inhibition alters PGE2-regulated ductus arteriosuscontractile pathways and prevents postnatal closure.ionoptix2019-10-22T18:26:17-05:00