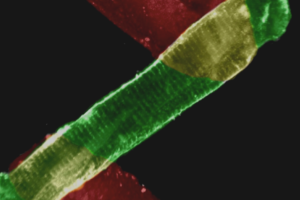
The transcription factor NFAT (nuclear factor of activated T-cells) is implicated in cardiac hypertrophy and vasculogenesis. NFAT activation, reflecting dephosphorylation by the calcium-dependent phosphatase, calcineurin, and subsequent nuclear localization, is generally thought to require a sustained increase in intracellular calcium. However, in smooth muscle we have found that elevation of calcium by membrane depolarization fails to induce an increase in nuclear localization of the NFATc3 isoform. Here, we demonstrate that physiological intravascular pressure (100 mm Hg) induces an increase in NFATc3 nuclear localization in mouse cerebral arteries. Pressure-induced NFATc3 nuclear accumulation is abrogated by endothelial denudation and by nitric-oxide synthase, cGMP-dependent kinase (PKG), and voltage-dependent calcium channels inhibition. We further show that exogenous nitric oxide, in combination with an elevation in calcium, is an effective stimulus for NFATc3 nuclear accumulation. c-Jun terminal kinase 2 (JNK) activity, which has been shown to regulate NFATc3 nuclear export, is also reduced by pressure, an effect that is prevented by pretreatment with a PKG inhibitor. Consistent with this, pressure-induced NFATc3 nuclear accumulation is independent of PKG in arteries from JNK2-/- mice. Collectively, our results indicate that both activation of the NO/PKG pathway and elevation of smooth muscle calcium are required for NFATc3 nuclear accumulation and that PKG inhibits JNK2 to decrease NFAT nuclear export. Our findings suggest that at physiological intravascular pressures NFATc3 is localized to the nucleus in smooth muscle cells of intact arteries and indicate a novel and unexpected role for nitric oxide/PKG in NFAT activation.
Intraluminal Pressure Is a Stimulus for NFATc3 Nuclear Accumulation: ROLE OF CALCIUM, ENDOTHELIUM-DERIVED NITRIC OXIDE, AND cGMP-DEPENDENT PROTEIN KINASEionoptix2019-10-22T18:24:32-05:00